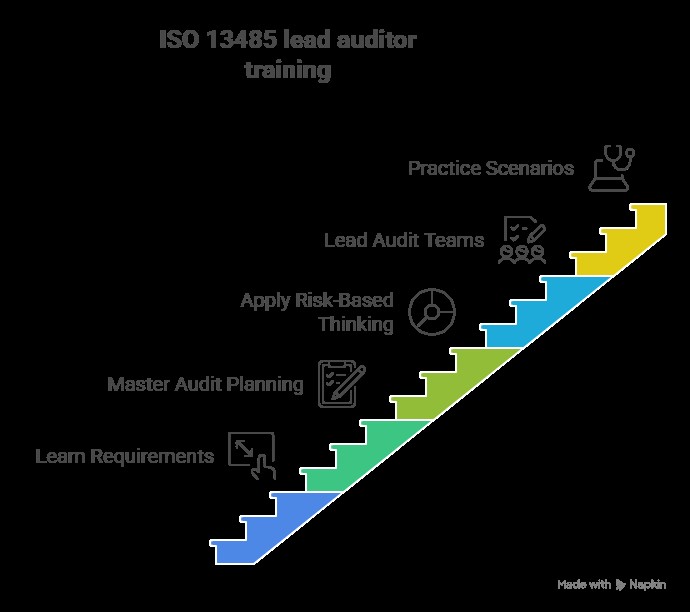
ISO 13485 lead auditor training prepares professionals to lead audits of medical device quality management systems. It covers ISO 13485 requirements, audit planning, execution, reporting, and risk-based assessment. Training enables auditors to identify nonconformities, recommend corrective actions, ensure regulatory compliance, and support continual improvement in medical device organizations.
https://iasiso-asia.com/VN/iso....-13485-lead-auditor-
علق
شارك





