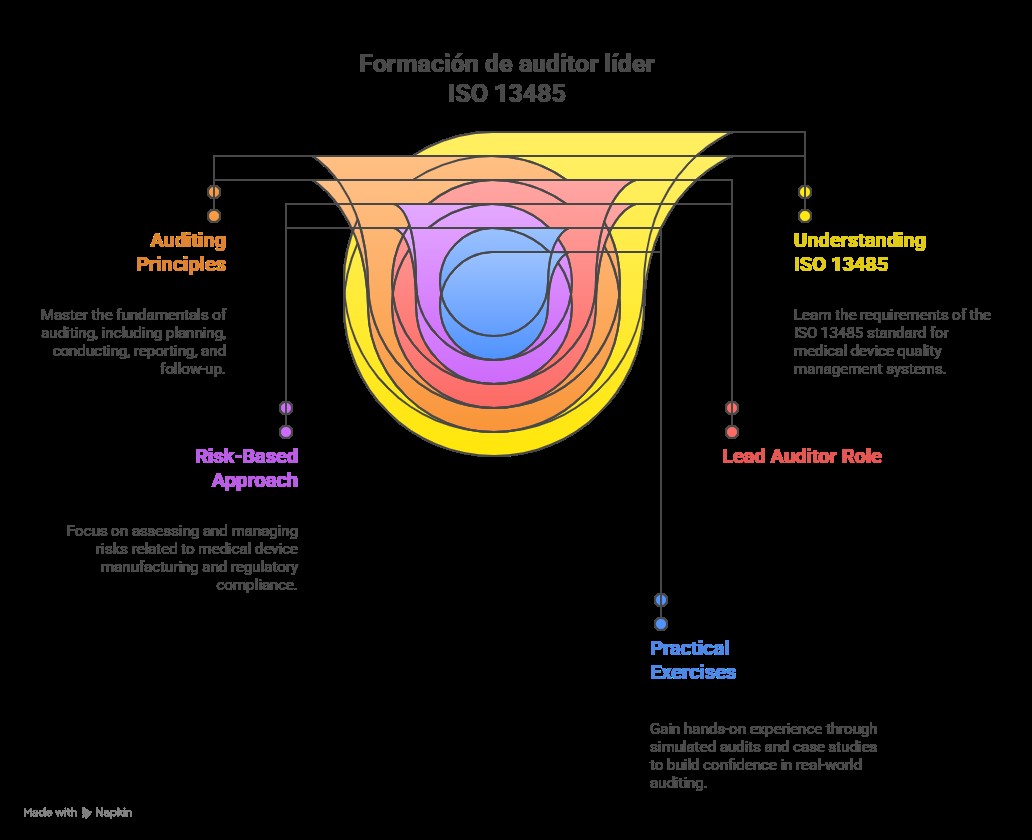
ISO 13485 Lead Auditor Training is a specialized program for professionals aiming to lead audits of quality management systems in the medical device industry. The training equips participants with skills to plan, conduct, and report audits, assess compliance with ISO 13485 standards, and identify risks in processes and procedures. It is ideal for auditors, quality managers, and regulatory professionals seeking to enhance expertise, ensure regulatory compliance, and promote product safety and quality.
https://iasiso-latinamerica.co....m/ar/iso-13485-lead-
Комментарий
Перепост





