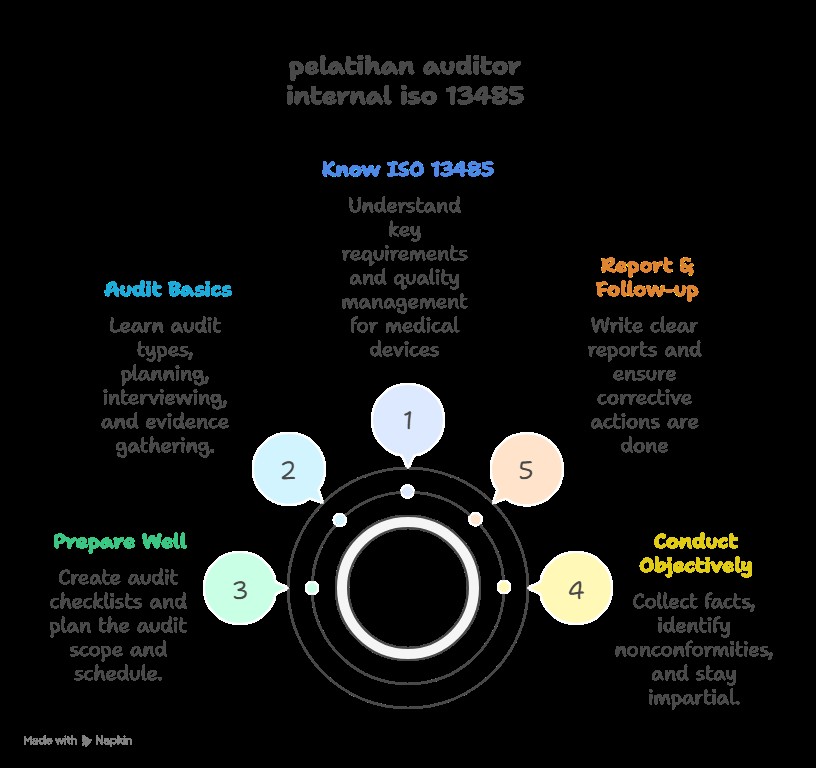
ISO 13485 Internal Auditor Training prepares participants to audit a medical device quality management system based on the ISO 13485 standard. It covers audit principles, techniques, and how to assess compliance to ensure the organization meets regulatory and quality requirements for medical device safety and effectiveness.
https://ias-indonesia.org/pela....tihan-auditor-intern
Kommentar
Delen





