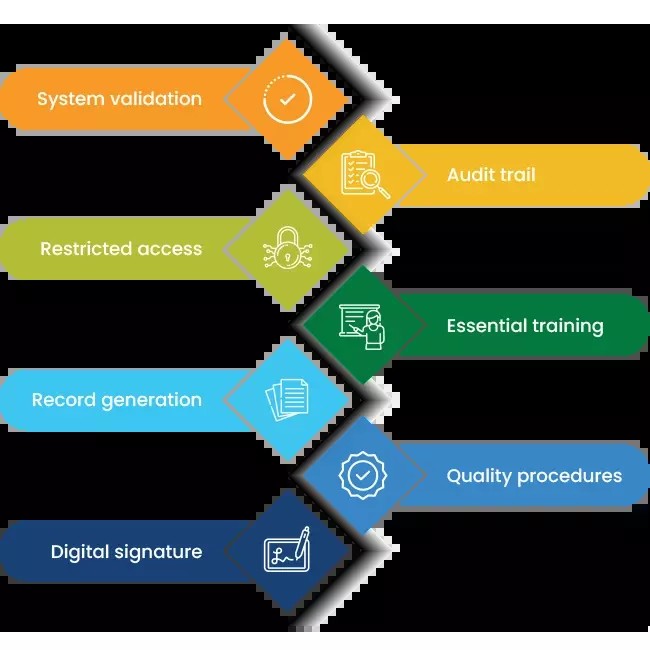
Key Requirements of 21 CFR Part 11
Organizations using closed electronic systems must meet FDA guidelines under 21 CFR Part 11.10 to ensure data authenticity, integrity, and confidentiality. Core requirements include:
i. System validation to ensure secure, reliable records
ii. Audit trails to track changes and processes
iii. Restricted access for authorized users only
iv. User training for proper system use
v. Searchable electronic records for easy retrieval
vi. Operational controls to manage people and processes
vii. Digital signatures to streamline approvals
For more Info, Please Visit: https://www.compliancequest.co....m/21-cfr-part-11-com
टिप्पणी
शेयर करना





